Proven Absorption
by Caco-2 Cell Line tests
thanks to the patented cutting-
edge technology lipoNIQ™
Liposomal Technology
is a technique used to encapsulate certain compounds, such as vitamins or other nutrients, in small spherical structures called liposomes. These liposomes are made up of a phospholipid bi-layer, similar to the structure of cell membranes, which enables them to be easily absorbed by the body. lipoNIQ™ is a cutting-edge technology to encapsulate nutrients in liposomes including an addidional polymer layer compared to standard liposomal technology and thus protect them better from the harsh digestive environment of the stomach and pass through the intestinal wall more easily, reaching the bloodstream intact. This may increase the amount of the nutrient that is available for the body to use, as well as reduce the likelihood of side effects associated with high doses of certain nutrients.
lipoNIQ™ is an advanced liposomal technology which is capable to develop liquid and dry liposomes with superior stability and high bioavailability.
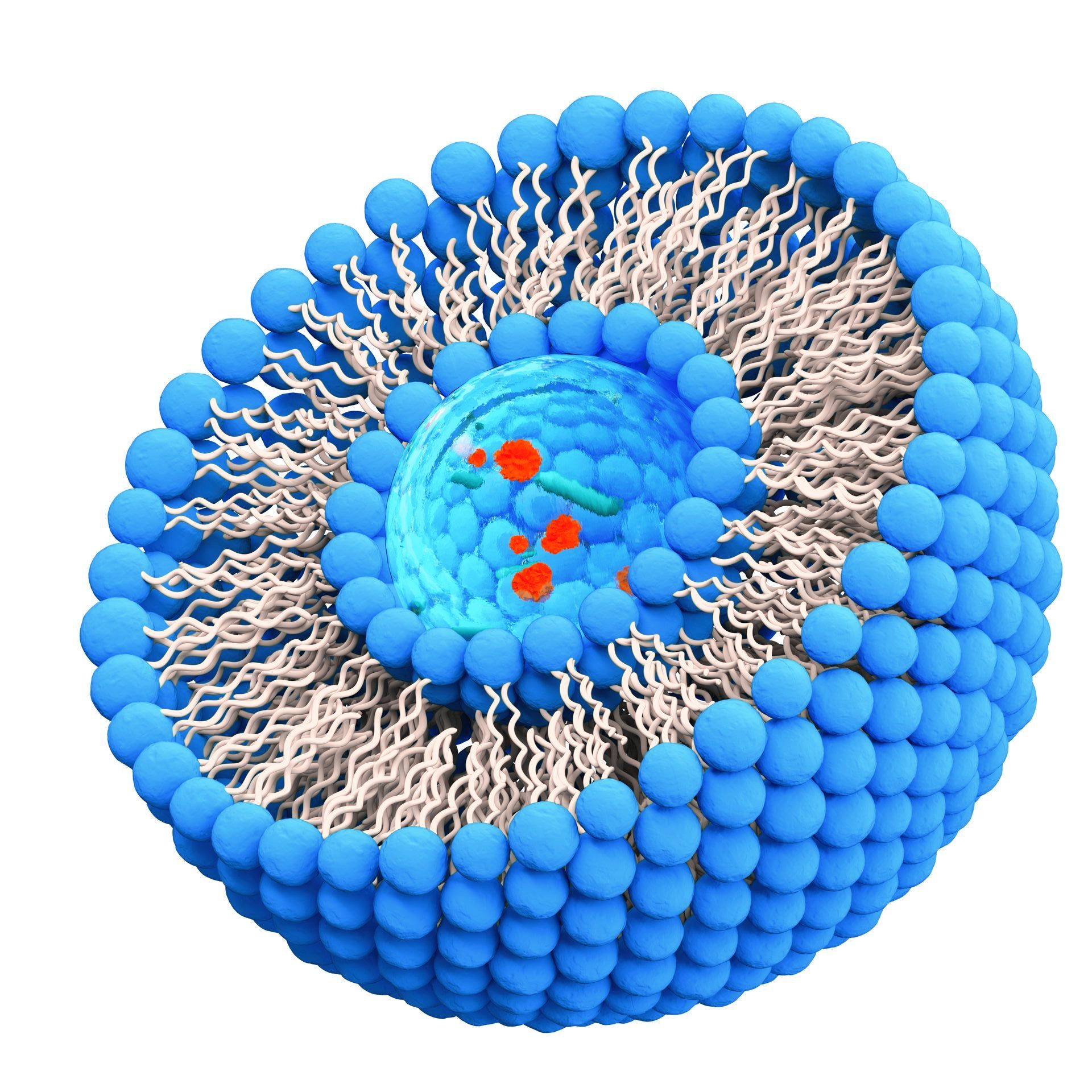
Liposomal Technologies
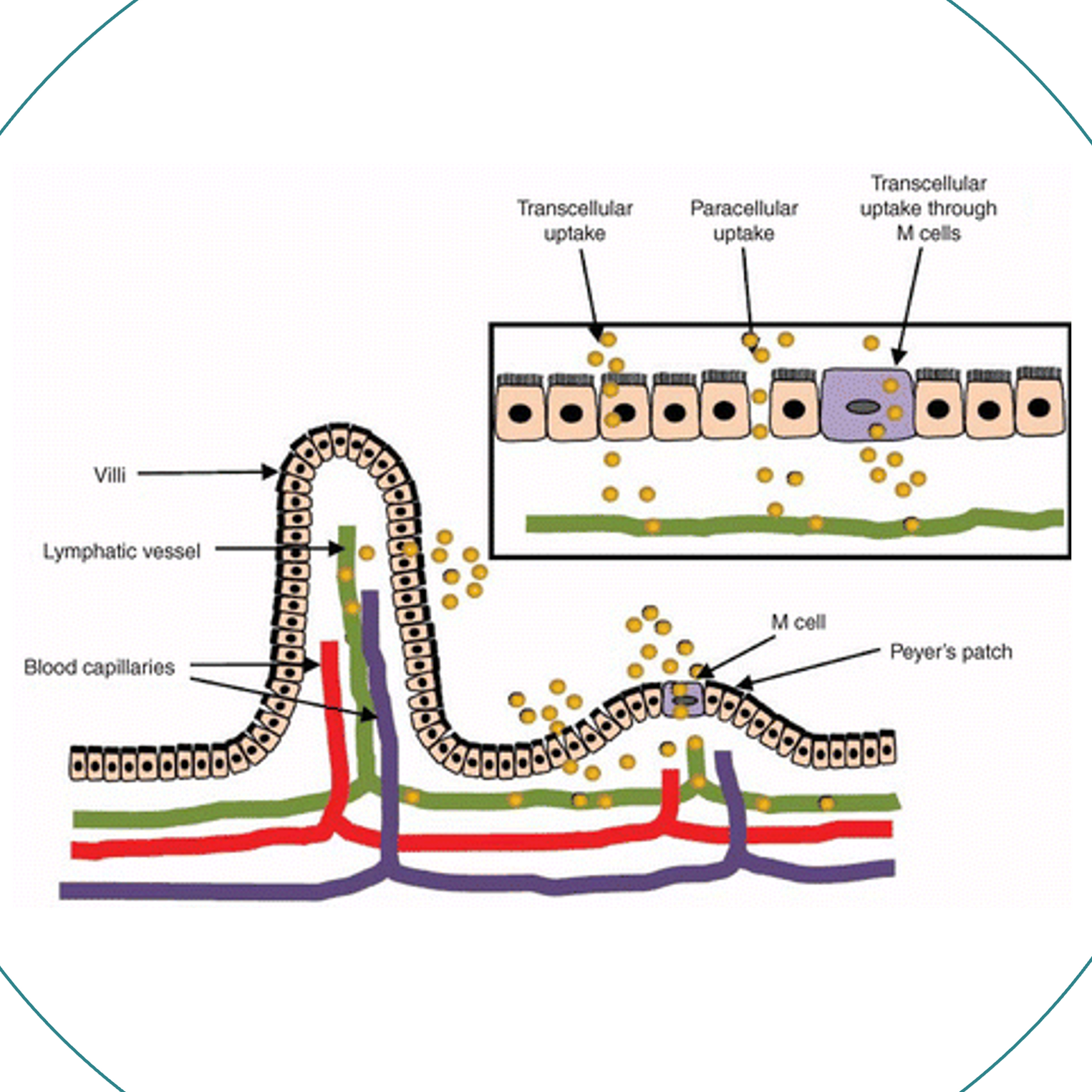
Absorption in Body
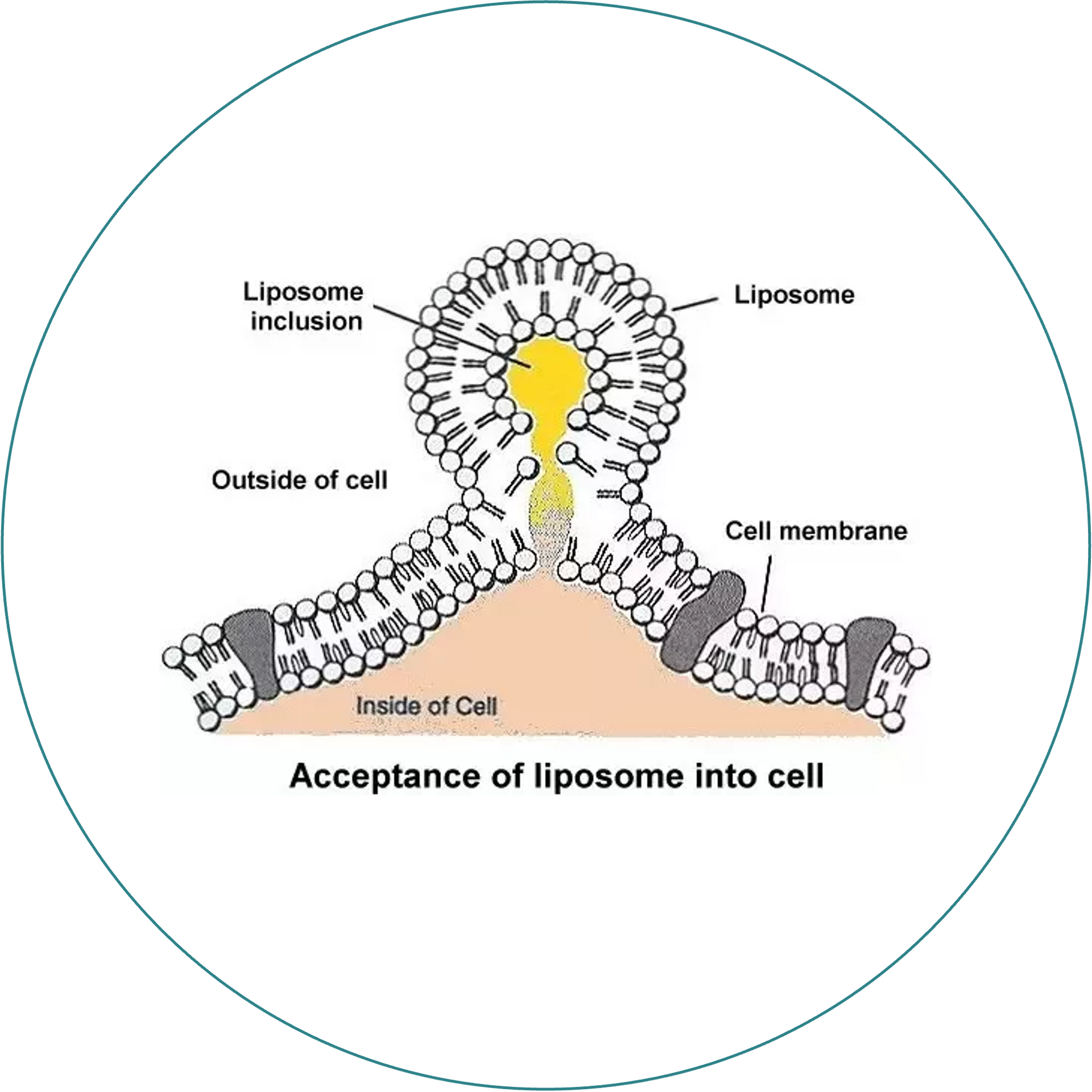
Cell Uptake
Liposomal encapsulation is a phenomenon that occurs in liquid milieu, the hydrodynamic forces are required to perform the encapsulation of the active ingredients with phospholipids.
The electrostatic potential (zeta potential) and the pressure of the liquid milieu helps to maintain the stability of the liposome. Therefore, prior to technology innovation, liposomes were thought to exist only in liquid form.
Recent developments in the liposomal encapsulation technologies succeeded to develop dry liposomes. lipoNIQ™
uses a technology of
double encapsulation of the active ingredients with phospholipids and natural polymers to achieve the required Zeta Potential values.
lipoNIQ™ is quality tested for its particle
size, zeta potential and gastric stability
Particle Size Analysis: Dynamic Light Scattering (DLS) result of lipoNIQ™ product - value between 150 - 400 nm defines superior quality and bioavailability
Zeta Potential result of lipoNIQ™ product. Value between 20 to 40 mV is important to block degradation of ingredient in GIS and thus provides superior bioavailability
Gastric Stability explained: Testing the liposome in different ph environments is necessary to proof the integrity of the liposome in the Gastro Intestinal System.
